As collagens age, they become increasingly crosslinked and insoluble - characteristics necessary for the structural role they play in tissues such as bone. Biocolor's Sircol™ INSOLUBLE Collagen Kit is an easy-to-use dye-binding assay that can be used to accurately measure the amount of insoluble collagen content in both in-vivo and in-vitro tissues.
Description
The Biocolor Sircol™ INSOLUBLE Collagen Assay Kit is a dye-binding assay designed for accurate quantification and measurement of insoluble or cross-linked collagen fibers. It is ideal for analyzing materials from sources such as tissues, bone, and calcified tissue.
The assay can be used to assess the rate of production of newly laid down collagen fibres during periods of rapid growth, development, tissue repair, remodeling and wound healing.
- It replaces the conventional hydroxyproline analysis. No more working with concentrated acids or alkalis!
- The Sircol Insoluble Assay uses mild acid and temperature treatment for 2 to 3 hours.
- The solubility conversion to denatured collagen and subsequent measurement can be completed within one working day.
- The ratio of soluble and insoluble collagens can be measured using the same Sircol Dye Reagent.
Principle of Method
Sircol™ Dye Reagent contains Sirius Red - a linear anionic dye with sulphonic acid side chain groups. Under assay conditions the Sircol dye binds the basic groups of soluble collagen molecules. Maximal binding occurs in collagens possessing intact triple helix organization as the highly ordered Gly-X-Yn helical structure of tropocollagen further contributes to dye binding. This results in a high degree of dye-collagen specificity. Affinity is progressively reduced during heat denaturation 4ºC due to the unwinding of the triple helix and formation of random chains.
Step 1. Samples being assayed for insoluble collagen must first undergo a 2-3 hour pre-treatment with Sircol Fragmentation reagent. This converts insoluble collagen into water-soluble gelatin can then be assayed.
Step 2. Addition of Sircol Dye Reagent to these pre-treated insoluble collagen samples results in the formation of a denatured collagen-dye complex. This complex then precipitates during the dye incubation period and is subsequently isolated by centrifugation, followed by washing to remove unbound dye. The Denatured collagen-bound dye is then eluted and measured spectrophotometrically.
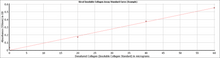
Step 3. The insoluble collagen content of unknown samples is quantified by comparison against a calibration curve prepared using a the denatured collagen standard supplied with the kit.

Specifications
| Product Category: | Extracellular Matrix Assays |
| Detection Method: | Colorimetric Detection (556 nm) |
| Assay Range: | 0 - 1000 µg/ml |
| Limit of Detection: | 100 µg/ml |
| Assay Run-Time: | 4 hours (including conversion of native insoluble collagen to soluble denatured collagen) |
| Type of Collagen Measured: | Covalent crosslinked insoluble collagen |
| Measurements Per Kit: | 110 in total (allows a maximum of 46 samples to be run in duplicate alongside a standard curve). |
| Storage (unopened kit): | Room Temperature (RT) |
| Manufacturer: | Biocolor Ltd. |
| Country of Origin: | United Kingdom |
Kit Contents
The standard size Sircol™ Insoluble Collagen Assay kit (cat. no. S2000) contains:
- Sircol Dye Reagent (1x 110 ml)
- Denatured Collagen Reference Standard (1x 5 ml, 1.0 mg/ml)
- Acid-Salt Wash Reagent (1x 20 ml)
- Fragmentation Reagent (1x 110 ml)
- Alkali Reagent (1x 110 ml)
- 2 ml screw-cap tubes for preparation of samples.
- Assay kit manual
Application Notes
Insoluble collagens must be converted into soluble form prior to assay. Instructions and reagents are provided with the kit. Depending on the sample, this will require prior salt/acid/acid-pepsin extraction.
Non-mammalian collagens may result in a reduced limit of detection. We recommend use of an assay standard matched to the non-mammalian species under assay.
Which Sircol Collagen Assay should I use for my application?

Resources
2024-2025 Product References
- Wen, Liru et al. “Establishment of an ex vivo cartilage damage model by combined collagenase treatment and mechanical loading.” Arthritis research & therapy vol. 27,1 30. 11 Feb. 2025, doi:10.1186/s13075-025-03499-7
- Dagnachew, Yinebeb Mezgebu et al. “Collagen deposition in lung parenchyma driven by depletion of interstitial Lyve-1+ macrophages prevents cigarette smoke-induced emphysema and loss of airway function.” Frontiers in immunology vol. 15 1493395. 3 Jan. 2025, doi:10.3389/fimmu.2024.1493395
- De Miguel-Gómez, Lucía et al. “Toward human uterus tissue engineering: Uterine decellularization in a non-human primate species.” Acta obstetricia et gynecologica Scandinavica vol. 104,3 (2025): 483-493. doi:10.1111/aogs.15030
- Choi, Soon Jin et al. “Increased efficiency of peripheral nerve regeneration using supercritical carbon dioxide-based decellularization in acellular nerve graft.” Scientific reports vol. 14,1 23696. 10 Oct. 2024, doi:10.1038/s41598-024-72672-w
- Mendibil, Unai et al. “Development of bioactive solid-foam scaffolds from decellularized cartilage with chondrogenic and osteogenic properties.” Materials today. Bio vol. 28 101228. 3 Sep. 2024, doi:10.1016/j.mtbio.2024.101228
- Kelly-Scumpia, Kindra M et al. “Modulating the extracellular matrix to treat wound healing defects in Ehlers-Danlos syndrome.” iScience vol. 27,9 110676. 6 Aug. 2024, doi:10.1016/j.isci.2024.110676
- Vikranth, Thirapurasundari et al. “Decellularisation and Characterisation of Porcine Pleura as Bioscaffolds in Tissue Engineering.” Journal of tissue engineering and regenerative medicine vol. 2024 9940673. 8 Jul. 2024, doi:10.1155/2024/9940673
- Chen, Te-An et al. “Engineering a robust and anisotropic cardiac-specific extracellular matrix scaffold for cardiac patch tissue engineering.” Matrix biology plus vol. 23 100151. 25 May. 2024, doi:10.1016/j.mbplus.2024.100151
- Harmon, Katrina A et al. “Varying Properties of Extracellular Matrix Grafts Impact Their Durability and Cell Attachment and Proliferation in an In Vitro Chronic Wound Model.” Journal of tissue engineering and regenerative medicine vol. 2024 6632276. 26 Apr. 2024, doi:10.1155/2024/6632276
- Martin, Meghan et al. “Differential Development of the Chordae Tendineae and Anterior Leaflet of the Bovine Mitral Valve.” Journal of cardiovascular development and disease vol. 11,4 106. 29 Mar. 2024, doi:10.3390/jcdd11040106
Usage
This product is intended for laboratory research use only and is not for use in diagnostic procedures.
Related Products
- Biocolor Sircol™ 2.0 Soluble Collagen Assay (96-well plate format)
- Biocolor Sircol™ Soluble Collagen Assay (original version)
- Collagen and Hydroxyproline Assays
- Extracellular Matrix Assays
- Extracellular Matrix Molecules
- Promed Bioscience high-purity collagen biomaterials
Ilex Life Sciences LLC is an official distributor of Biocolor products.