NOTE: The new Biocolor Sircol™ 2.0 Soluble Collagen Assay is now available! This latest addition to the highly cited family of "Sircol" collagen kits combines an
updated 96-well microplate format with an improved dye formulation to significantly enhance collagen sensitivity and specificity. This makes it possible to directly analyze collagen from complex samples such as conditioned media during 2D or 3D cell culture.
We recommend that new customers use our Sircol 2.0 assay kit as it improves sensitivity, specificity, and user-friendliness; however, we will continue to offer the older, "original" kit as well.
Description
The Biocolor Sircol™ Soluble Collagen Assay is a quantitative dye-binding assay for measurement of both acid-soluble and pepsin-soluble collagens.
The assay can assess the rate of newly synthesized collagen. New collagen is produced during periods of rapid growth and development. New collagen is also generated during inflammation, wound healing and tumor development.
The Sircol Assay can monitor collagen produced in situ, or during in-vitro cell culture. It can also measure in-vitro extracellular matrix formation.
This assay is not suitable for covalent cross-linked collagen. However, the Sircol Insoluble Collagen Assay (S2000) replaces hydroxyproline analysis. This allows the customer to measure insoluble collagen within one working day.
Principle of Method
Sircol™ Dye Reagent contains Sirius Red - a linear anionic dye with sulphonic acid side chain groups. Under assay conditions the Sircol dye binds the basic groups of soluble collagen molecules. Maximal binding occurs in collagens possessing intact triple helix organization as the highly ordered Gly-X-Yn helical structure of tropocollagen further contributes to dye binding. This results in a high degree of dye-collagen specificity. Affinity is progressively reduced during heat denaturation 4ºC due to the unwinding of the triple helix and formation of random chains.
Step 1. Addition of Sircol Dye Reagent to samples containing soluble collagen results in the formation of a collagen-dye complex. This complex then precipitates during the dye incubation period and is subsequently isolated by centrifugation, followed by washing to remove unbound dye.
Step 2. Collagen-bound dye is then eluted and measured spectrophotometrically.
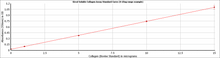
Step 3. The collagen content of unknown samples is quantified by comparison against a calibration curve prepared using a collagen standard supplied with the kit.

Specifications
| Product Category: | Extracellular Matrix Assays |
| Detection Method: | Colorimetric Detection (556 nm) |
| Assay Range: | 0 - 150 µg/ml or 0 - 500 µg/ml (depending on setup) |
| Limit of Detection: | 10 µg/ml |
| Assay Run-Time: | 1.5 hours |
| Sensitivity: | The sensitivity of the Sircol Soluble Collagen Assay is 1.0 µg collagen. Quantities as low as 1.0 µg of collagen can be recovered from a 1 ml volume if the Collagen Isolation & Concentration protocol is used (See Sircol assay manual for more detail). |
| Type of Collagen Measured: |
|
| Measurements Per Kit: | 110 in total (allows a maximum of 48 samples to be run in duplicate alongside a standard curve). |
| Storage (unopened kit): | Room Temperature (RT) |
| Manufacturer: | Biocolor Ltd. |
| Country of Origin: | United Kingdom |
Suitable Samples
Soluble* collagens from mammalian sources**:
-
In-vivo: tissues, cartilages, fluids.
-
In-vitro: 2D/3D culture extracellular matrices. Customers wishing to analyze conditioned media should first consider the new Sircol 2.0 assay kit.
* Collagens must be in soluble form, depending on sample this will require prior salt/acid/acid-pepsin extraction.
** Non-mammalian collagens may result in a reduced limit of detection. We recommend use of a collagen standard matched to the non-mammalian species under assay.
Many customers have found that the straightforward sample processing and analysis of Sircol make it a good alternative to conventional hydroxyproline analysis.
Kit Contents
The standard size Sircol™ Soluble Collagen Assay kit (cat. no. S1000) contains:
- Sircol Dye Reagent (1x 110 ml)
- Collagen Reference Standard (1x 5 ml, 0.5 mg/ml soluble Bovine collagen)
- Acid-Salt Wash Reagent (1x 20 ml)
- Collagen Isolation and concentration Reagent (1x 20 ml)
- Alkali Reagent (1x 110 ml)
- Acid-Neutralising Reagent (1x 20 ml)
- 1.5 ml micro-centrifuge tubes for dye-labelling reaction.
- Assay kit manual
Which Sircol Collagen Assay should I use for my application?

Resources
2025 Product References
- Yu, Sheeline et al. “Cyclic GMP-AMP synthase expression is enhanced in systemic sclerosis-associated interstitial lung disease and stimulates inflammatory myofibroblast activation.” The European respiratory journal vol. 66,2 2401564. 8 Aug. 2025, doi:10.1183/13993003.01564-2024
- Zhou, Jialiang et al. “Decellularized Adipose Matrix Rejuvenates Photoaged Skin through Immune Microenvironment Modulation.” BME frontiers vol. 6 0166. 4 Aug. 2025, doi:10.34133/bmef.0166
- Jin, Zhengxin et al. “Pantothenic acid ameliorates hepatic fibrosis by targeting IGFBP6 to regulate the TGF-β/SMADs pathway.” Communications biology vol. 8,1 1127. 29 Jul. 2025, doi:10.1038/s42003-025-08527-5
- Song, Minwoo et al. “A Hierarchical Short Microneedle-Cupping Dual-Amplified Patch Enables Accelerated, Uniform, Pain-Free Transdermal Delivery of Extracellular Vesicles.” Nano-micro letters vol. 18,1 11. 23 Jul. 2025, doi:10.1007/s40820-025-01853-7
- Hong, Seung Hee et al. “Photodynamically tunable ROS-generating hydrogels for accelerated tissue regeneration.” Bioactive materials vol. 51 977-992. 8 Jul. 2025, doi:10.1016/j.bioactmat.2025.05.006
- Kim, Hyunsik et al. “Loss of p300 in proximal tubular cells reduces renal fibrosis and endothelial-mesenchymal transition.” EMBO molecular medicine vol. 17,7 (2025): 1575-1598. doi:10.1038/s44321-025-00243-1
- Ntinopoulou, Maria et al. “IL-1b-Bearing NETs: Bridging Inflammation to Early Cirrhosis in Hepatitis B.” International journal of molecular sciences vol. 26,12 5733. 15 Jun. 2025, doi:10.3390/ijms26125733
- Bozzini, Sara et al. “Hyaluronic Acid‐Decorated Liposomes for the Intrapulmonary Delivery of Imatinib: A Targeted Treatment for Postinflammatory Pulmonary Fibrosis.” Small Science vol. 5,8 2500144. 10 Jun. 2025, doi:10.1002/smsc.202500144
- Vera, Renzo E et al. “Paracrine regulation of pancreatic cancer cell response to chemotherapy by GLI2-collagen I signaling.” The Journal of biological chemistry vol. 301,7 (2025): 110311. doi:10.1016/j.jbc.2025.110311
- Hussein, Kamal H et al. “Efficacy of xenogeneic fresh and lyophilized amniotic membranes on the healing of experimentally induced full-thickness skin wounds in dogs.” Scientific reports vol. 15,1 15605. 4 May. 2025, doi:10.1038/s41598-025-95023-9
- Tang, Chang et al. “Molecular Hydrogen Ameliorates Anti-Desmoglein 1 Antibody-Induced Pemphigus-Associated Interstitial Lung Disease by Inhibiting Oxidative Stress.” International journal of molecular sciences vol. 26,9 4203. 28 Apr. 2025, doi:10.3390/ijms26094203
- Zhang, Sixuan et al. “Probiotics promote cellular wound healing responses by modulating the PI3K and TGF-β/Smad signaling pathways.” Cell communication and signaling : CCS vol. 23,1 195. 23 Apr. 2025, doi:10.1186/s12964-025-02179-y
- Xu, Yan et al. “CBD-conjugated BMP-inhibiting exosomes on collagen scaffold dual-target Achilles tendon repair: Synergistic regeneration and heterotopic ossification prevention.” Materials today. Bio vol. 32 101790. 22 Apr. 2025, doi:10.1016/j.mtbio.2025.101790
- Xie, Yuanyuan et al. “Identifying TNFSF4low-MSCs superiorly treating idiopathic pulmonary fibrosis through Tregs differentiation modulation.” Stem cell research & therapy vol. 16,1 194. 20 Apr. 2025, doi:10.1186/s13287-025-04313-6
- Shi, Qingqiang et al. “MFGE8 regulates the EndoMT of HLMECs through the BMP signaling pathway and fibrosis in acute lung injury.” Respiratory research vol. 26,1 142. 13 Apr. 2025, doi:10.1186/s12931-025-03215-8
- Srakhao, Waritorn et al. “Therapeutic Potential of Cannabidiol Cyclodextrin Complex in Polymeric Micelle and Tetrahydrocurcumin Cyclodextrin Complex Loaded in Hydrogel to Treat Lymphedema.” International journal of molecular sciences vol. 26,7 3428. 6 Apr. 2025, doi:10.3390/ijms26073428
- Tsilingiris, Dimitrios et al. “Interleukin-8/Matrix Metalloproteinase-9 Axis Impairs Wound Healing in Type 2 Diabetes through Neutrophil Extracellular Traps-Fibroblast Crosstalk.” European journal of immunology vol. 55,4 (2025): e202451664. doi:10.1002/eji.202451664
- Bakhos, Jules Joel et al. “Inhibiting atrial natriuretic peptide clearance reduces myocardial fibrosis and improves cardiac function in diabetic rats.” European heart journal open vol. 5,2 oeaf031. 19 Mar. 2025, doi:10.1093/ehjopen/oeaf031
- Shi, Xiangguang et al. “Increased melanin induces aberrant keratinocyte - melanocyte - basal - fibroblast cell communication and fibrogenesis by inducing iron overload and ferroptosis resistance in keloids.” Cell communication and signaling : CCS vol. 23,1 141. 18 Mar. 2025, doi:10.1186/s12964-025-02116-z
- Zhang, Zejin et al. “PD-1 inhibition disrupts collagen homeostasis and aggravates cardiac dysfunction through endothelial-fibroblast crosstalk and EndMT.” Frontiers in pharmacology vol. 16 1549487. 17 Mar. 2025, doi:10.3389/fphar.2025.1549487
- Banaschewski, Brandon J H et al. “Emergence of inflammatory fibroblasts with aging in Hermansky-Pudlak syndrome associated pulmonary fibrosis.” Communications biology vol. 8,1 284. 22 Feb. 2025, doi:10.1038/s42003-025-07589-9
- Wong, Keng Lin et al. “Mesenchymal Stem Cell Exosome and Fibrin Sealant Composite Enhances Rabbit Anterior Cruciate Ligament Repair.” The American journal of sports medicine vol. 53,4 (2025): 871-884. doi:10.1177/03635465241313142
- Chen, Shuang et al. “Exploring the correlation between magnetic resonance diffusion tensor imaging (DTI) parameters and aquaporin expression and biochemical composition content in degenerative intervertebral disc nucleus pulposus tissue: a clinical experimental study.” BMC musculoskeletal disorders vol. 26,1 157. 17 Feb. 2025, doi:10.1186/s12891-025-08382-9
- Besio, Roberta et al. “The administration of exogenous HSP47 as a collagen-specific therapeutic approach.” JCI insight vol. 10,6 e181570. 6 Feb. 2025, doi:10.1172/jci.insight.181570
- Kim, Jungbum et al. “Lung-homing nanoliposomes for early intervention in NETosis and inflammation during acute lung injury.” Nano convergence vol. 12,1 8. 3 Feb. 2025, doi:10.1186/s40580-025-00475-4
- Sekiguchi, Akiko et al. “Inhibition of skin fibrosis via regulation of Th17/Treg imbalance in systemic sclerosis.” Scientific reports vol. 15,1 1423. 9 Jan. 2025, doi:10.1038/s41598-025-85895-2
- McCall, A Scott et al. “Hypoxia-inducible factor 2 regulates alveolar regeneration after repetitive injury in three-dimensional cellular and in vivo models.” Science translational medicine vol. 17,780 (2025): eadk8623. doi:10.1126/scitranslmed.adk8623
- Qiu, Huijun et al. “Human Umbilical Cord-Mesenchymal Stem Cells Combined With Low Dosage Nintedanib Rather Than Using Alone Mitigates Pulmonary Fibrosis in Mice.” Stem cells international vol. 2025 9445735. 7 Jan. 2025, doi:10.1155/sci/9445735
- Dagnachew, Yinebeb Mezgebu et al. “Collagen deposition in lung parenchyma driven by depletion of interstitial Lyve-1+ macrophages prevents cigarette smoke-induced emphysema and loss of airway function.” Frontiers in immunology vol. 15 1493395. 3 Jan. 2025, doi:10.3389/fimmu.2024.1493395
Usage
This product is intended for laboratory research use only and is not for use in diagnostic procedures.
Related Products
- NEW: Biocolor Sircol™ 2.0 Soluble Collagen Assay
- Sircol 2.0™ uses an updated 96-well microplate format with an improved dye formulation to significantly enhance collagen sensitivity and specificity.
- Biocolor Sircol™ Insoluble Collagen Assay
- Collagen and Hydroxyproline Assays
- Extracellular Matrix Assays
- Extracellular Matrix Molecules
- Promed Bioscience high-purity collagen biomaterials
Ilex Life Sciences LLC is an official distributor of Biocolor products.