Description
Quantitative measurement of total protein in acid hydrolysates of fresh, frozen, fixed and paraffin embedded tissues in a fast and simple 96-well plate format!
The QuickZyme Total Collagen Assay or QuickZyme Hydroxyproline Assay are frequently used to determine the amount of collagen in biological samples such as tissues, tissue extracts, cell extracts, and culture media. For the proper interpretation of the data obtained these should be compared to some reference quantity like wet- or dry- weight of the tissue, amount of protein, DNA etc. Some of these quantities are not easy to measure, or require an additional sample because they cannot be measured directly in acid hydrolysates. It would be helpful if it was possible to determine such a reference in the acid hydrolysate also used for determination of total collagen or hydroxyproline, such that no longer additional samples or cumbersome methods would be required. The QuickZyme Total Protein Assay offers a convenient solution in such cases.
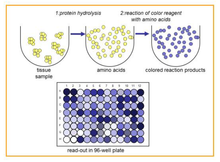
In this assay the total amount of amino acids present in the same acid hydrolysate used in the QuickZyme Total Collagen and Hydroxyproline Assays is easily determined. This total amount of amino acids in the hydrolysate is a direct measure of the amount of protein present in the original sample. Combination of the QuickZyme Total Protein Assay with either the Total Collagen or Hydroxyproline Assay allows the expression of data as collagen or hydroxyproline per total amount of protein.
The QuickZyme Total Protein Assay is based on the formation of a blue colored product from genipin with free amino acids (Lee et al. Anal. Chim. Acta 480(2003)267; Fujikawa et al. J. Ferment. Technol. 65(1987)149). The assay measures the total amount of amino acids present in the hydrolyzed sample, with exception of proline and hydroxyproline. The amount of amino acids is a good measure for the amount of protein. A hydrolyzed protein standard is supplied with the kit to directly convert data to mg of protein.
The assay is simple and results in a colored product with an absorbance maximum close to 570 nm. The assay is developed to measure protein in acid tissue and/or protein hydrolysates in such a way that the assay can be performed directly on the hydrolysate without a drying or neutralization step. When used in addition to the QuickZyme Total Collagen Assay or QuickZyme Hydroxyproline Assay, this assay kit allows for easy expression of the collagen or hydroxyproline amounts per total amount of protein.
Features
- Quantitative measurement of protein in acid hydrolysates
- Species independent
- Colorimetric read out at 570 nm
- Broad dynamic range: 0.05 to 3.0 mg/ml. Sensitivity: 0.007 mg/ml
- Uses BSA as standard
- Ease-of-use: faster and easier than ELISA
- Storage at room temperature
- This kit does not require special equipment
Type
Colormetric Assay

Applications
- Tissue extract, acid hydrolysates of tissues, Conditioned media, Cells/cell extracts, Frozen or paraffin tissue sections
Sensitivity
0.007 mg/ml
Quantitative Measurement

A typical protein standard curve in the range of 0.047-3.00 mg/ml protein, the background is not subtracted).
Storage
Unopened kit: Store at 4°C in the dark. Do not use kit components past kit expiration date.
Opened kit / reconstituted reagents: The reconstituted reagents should be stored light protected at 4°C (< 1 week), longtime storage at -20°C (stable for at least 2 months). The diluted color reagent solution should be prepared fresh and used the same day.
Resources
- Product Datasheet (PDS)
- Application note 1: How to choose your collagen assay?
- Application note 2: How to measure collagen in mouse tissues?
- Application note 3: Collagen per protein analysis in fresh, frozen and formalin-fixed tissues
- Application note 4: Collagen analysis in fish tissue
Product References
- Tarnutzer, Katharina et al. “Collagen constitutes about 12% in females and 17% in males of the total protein in mice.” Scientific reports vol. 13,1 4490. 18 Mar. 2023, doi:10.1038/s41598-023-31566-z
- Gart, Eveline et al. “Translational characterization of the temporal dynamics of metabolic dysfunctions in liver, adipose tissue and the gut during diet-induced NASH development in Ldlr-/-.Leiden mice.” Heliyon vol. 9,3 e13985. 24 Feb. 2023, doi:10.1016/j.heliyon.2023.e13985
- Peng, Wei et al. “Activin A and CCR2 regulate macrophage function in testicular fibrosis caused by experimental autoimmune orchitis.” Cellular and molecular life sciences : CMLS vol. 79,12 602. 24 Nov. 2022, doi:10.1007/s00018-022-04632-4
- Jaeger, Benedikt et al. “Airway basal cells show a dedifferentiated KRT17highPhenotype and promote fibrosis in idiopathic pulmonary fibrosis.” Nature communications vol. 13,1 5637. 26 Sep. 2022, doi:10.1038/s41467-022-33193-0
- Seidel, Florine et al. “Therapeutic Intervention with Anti-Complement Component 5 Antibody Does Not Reduce NASH but Does Attenuate Atherosclerosis and MIF Concentrations in Ldlr-/-.Leiden Mice.” International journal of molecular sciences vol. 23,18 10736. 14 Sep. 2022, doi:10.3390/ijms231810736
- Choi, Sung-Eun et al. “Inhibition of lysyl oxidase‑like 2 ameliorates folic acid‑induced renal tubulointerstitial fibrosis.” Experimental and therapeutic medicine vol. 24,5 648. 2 Sep. 2022, doi:10.3892/etm.2022.11585
- Brereton, Christopher J et al. “Pseudohypoxic HIF pathway activation dysregulates collagen structure-function in human lung fibrosis.” eLife vol. 11 e69348. 21 Feb. 2022, doi:10.7554/eLife.69348
- Morrison, Martine C et al. “Heat-Inactivated Akkermansia muciniphila Improves Gut Permeability but Does Not Prevent Development of Non-Alcoholic Steatohepatitis in Diet-Induced Obese Ldlr-/-.Leiden Mice.” International journal of molecular sciences vol. 23,4 2325. 19 Feb. 2022, doi:10.3390/ijms23042325
- Gart, Eveline et al. “Butyrate Protects against Diet-Induced NASH and Liver Fibrosis and Suppresses Specific Non-Canonical TGF-β Signaling Pathways in Human Hepatic Stellate Cells.” Biomedicines vol. 9,12 1954. 20 Dec. 2021, doi:10.3390/biomedicines9121954
- Kelly, Shannon C et al. “The right ventricular transcriptome signature in Ossabaw swine with cardiometabolic heart failure: implications for the coronary vasculature.” Physiological genomics vol. 53,3 (2021): 99-115. doi:10.1152/physiolgenomics.00093.2020
- Schneider, Jay W et al. “Dysregulated ribonucleoprotein granules promote cardiomyopathy in RBM20 gene-edited pigs.” Nature medicine vol. 26,11 (2020): 1788-1800. doi:10.1038/s41591-020-1087-x
- Zou, Jun et al. “Stabilization of hypoxia-inducible factor ameliorates glomerular injury sensitization after tubulointerstitial injury.” Kidney international vol. 99,3 (2021): 620-631. doi:10.1016/j.kint.2020.09.031
- Zhang, Mingjun et al. “Targeting the Wnt signaling pathway through R-spondin 3 identifies an anti-fibrosis treatment strategy for multiple organs.” PloS one vol. 15,3 e0229445. 11 Mar. 2020, doi:10.1371/journal.pone.0229445
- Ono, Hiroya et al. “AMPK Complex Activation Promotes Sarcolemmal Repair in Dysferlinopathy.” Molecular therapy : the journal of the American Society of Gene Therapy vol. 28,4 (2020): 1133-1153. doi:10.1016/j.ymthe.2020.02.006
- Jin, Heng et al. “Innate Immune Signaling Contributes to Tubular Cell Senescence in the Glis2 Knockout Mouse Model of Nephronophthisis.” The American journal of pathology vol. 190,1 (2020): 176-189. doi:10.1016/j.ajpath.2019.09.013
- Kim, David H et al. “Calpain 9 as a therapeutic target in TGFβ-induced mesenchymal transition and fibrosis.” Science translational medicine vol. 11,501 (2019): eaau2814. doi:10.1126/scitranslmed.aau2814
- Landolt, Lea et al. “AXL targeting reduces fibrosis development in experimental unilateral ureteral obstruction.” Physiological reports vol. 7,10 (2019): e14091. doi:10.14814/phy2.14091
- Vaisman, Boris L et al. “LCAT Enzyme Replacement Therapy Reduces LpX and Improves Kidney Function in a Mouse Model of Familial LCAT Deficiency.” The Journal of pharmacology and experimental therapeutics vol. 368,3 (2019): 423-434. doi:10.1124/jpet.118.251876
- Jin, Heng et al. “Epithelial innate immunity mediates tubular cell senescence after kidney injury.” JCI insight vol. 4,2 e125490. 24 Jan. 2019, doi:10.1172/jci.insight.125490
- Teuscher, Alina C et al. “Assessing Collagen Deposition During Aging in Mammalian Tissue and in Caenorhabditis elegans.” Methods in molecular biology (Clifton, N.J.) vol. 1944 (2019): 169-188. doi:10.1007/978-1-4939-9095-5_13
- Schilter, Heidi et al. “The lysyl oxidase like 2/3 enzymatic inhibitor, PXS-5153A, reduces crosslinks and ameliorates fibrosis.” Journal of cellular and molecular medicine vol. 23,3 (2019): 1759-1770. doi:10.1111/jcmm.14074
- Jones, Mark G et al. “Nanoscale dysregulation of collagen structure-function disrupts mechano-homeostasis and mediates pulmonary fibrosis.” eLife vol. 7 e36354. 3 Jul. 2018, doi:10.7554/eLife.36354
- VeDepo, Mitchell et al. “Comparison of Candidate Cell Populations for the Recellularization of Decellularized Heart Valves.” Cellular and molecular bioengineering vol. 11,3 197-209. 30 Apr. 2018, doi:10.1007/s12195-018-0524-0
Related Products
- QuickZyme Total Collagen Assay
- QuickZyme Hydroxyproline Assay
- QuickZyme Sensitive Tissue Collagen Assay
- QuickZyme Sensitive Tissue Hydroxyproline Assay
- Extracellular Matrix Assays
Ilex Life Sciences LLC is an official distributor of QuickZyme Biosciences.